Cervical Cancer Strikes Back
Lecturer: Prof.Dr. Olivera Markovic, President Global Academy for Women’s health, Inc. andDirector, BioSciCon, Inc.
Introduction
Cervical cancer is known from ancient times as the important killer of women from malignant diseases. For thousands of years this disease is resistant to any therapy except to an early removal of suspect lesions, and in recent two Centuries, following the increase in the world population and better communication enabling interactions between different people, this fatal disease has gained recognition of having natural grown of mortality rate for about 10% per year.
In the middle of 20th Century, the mortality of cervical cancer worldwide and in the US was about 30 per hundred thousands. In countries with any type of screening this number was reduced to one third, and in the US, the introduction and nationwide application of Pap test screening has helped American healthcare providers to further reduce the mortality rate to about 3 per a hundred thousand women, reduction of more than 85%. It was expected that with all new improvements (LBP, HPV) mortality will be reduced to less than 1 per a hundred thousand women.
Unfortunately, it did not happen. Now, in 2019, the mortality of cervical cancer in the US is back to the best results obtained with the classic Pap test only, or 3 per thousand. CDC has recently reported that new incidence of cervical cancer in the US is predicted to be 13,200 for 2019, or about 4000 higher than in the best period before. Obviously cervical cancer is present and active. TheACS has published their newest guidelines recovering the Pap test and cytological screening to protect the accuracy of prevention. Hologic and Quest have followed this pattern and are reviving the cytological screening. Even Roche, the manufacturer of COBAS is mentioning the value of microscopic analyses and cyto-diagnosis.
What happened? Something went wrong. But what? What to do to prevent errors to repeat, and to keep the trend for reduction of mortality as predicted.
We went back to the history of the Pap test and, we think, we were able to identify some periods when application of Pap test was exposed to changes that affected its successful progression as the “best cancer screening test available.”
1. Early period – 1945 – 1996
In 20th Century, American gynecologists used vaginal fluids to collect exfoliated squamous cells to make vaginal smears and to search for cervical cancer. It was a successful approach reducing the mortality, but was not accurate method.
In 1945 Dr. George Papanicolaou introduced his test using cleaning cervix and then excoriating cervical superficial cells. It was much better technique providing cleaner smears and better vision of cellular structures enabling the investigators to detect suspicious cells that could develop into cervical cancer. The basic cytological classification: no changes, mild changes, intermediate and severe changes ending with cervical cancer, was established and served the purpose for years. In this period, by sending women with positive test to colposcopy and further diagnosis including removal of suspicious lesions, American health care providers reduced further the cervical cancer mortality rate to 3 per thousand, the rate that was sufficient to remove the cervical cancer from the list of 10 most frequent cause of death ofAmerican women.
2. The Bethesda NIH Consensus Conference on Cervical Cancer: TBS –surrogate classification. Twenty percent false negatives. Call for improvement.
However, in this “golden “ period for Pap test, several cytopathology laboratories lost legal suites for medical malpractice because they missed to identify false negative Pap smears of women who died of cervical cancer although being diagnosed as negative Pap.
In the meantime, scientific information accrued that the standard Pap test, in a form as applied, might have about 20% false negative readings due to problems with specimen collection options, specimen crowded with infectious cells, and difficulty for clear reading of DNA changes.
The National Consensus Conference was called to resolve those problems with consensus if not with pure evidence.
The Conference ended with a success.
Pap smear test was confirmed as “the best cancer screening test available”
The 20% false negative rate was allocated to the specimen collection
The call was open for new technology to improve this insufficiency.
Finally, the Bethesda System classification was developed to change the standard cytological classification with a modern version using more strange words: nil (no disease), BCC (benign cervical cell changes including reactive);ASCUS ) abnormal squamous cells with undetermined significance; LSIL (Low grade squamous intraepithelial lesion); HSIL (High grade cervical intraepithelial lesion) and ICC (invasive cervical carcinoma).
Although this new classification looks much better I(more precise] than the old one, it really is only a surrogate list of endpoints to rank the success/failure of the new methods for cervical cancer screening. The robust, outcome based (life/death) method was put aside. This was the fist change affecting measuring the accuracy of the mortality rate caused by cervical cancer.
3. Liquid based specimen collection
Soon ThinPrep Pap test was introduced by Cytyc Corp., and SurePath Pap test by TriPath Imaging Inc. Both are based on the specimens collected in their special cell preservative solution and their apparatus (processor) for automatic slide preparation before staining. Papanicolaou stains remained unchanged.
Both companies brought slides very much appealing to cytopathologists –they were free of detritus, reduced number of small inflammatory cells, squamous cell much better distributed over the slide, transparency preserved and intracellular components clearly visible. It is easy to diagnose TBS changes.
Although both companies advertised that their solution contains much more cells from the sample, what is true, in practice, not frequently more than one slide is processed. Using another sample will rocket the price per investigation. The cost for Pap test exam has already doubled with trend to rise.
The solution was found in reduction of sampling frequency (once in three years). This was another, but very serious damage to the reduction of the false negative rates. Obviously, it was a nonrealistic solution.
4. Rising cost – extension of mandatory periods for screening
Annual screening was recommended to reduce 20% false negative rates.Within three years this rate could be reduced to 5% or the acceptable statistical error rate. Extending the periods between screenings is affecting false negative rate negatively – increasing the probability of false results to up to 50% which is unacceptable for any test.
5. HPV enthusiasm for eradication of the virus etiology
In the beginning of 21st Century the HPV has arrived. Indeed, it was approved as test to detect HPV virus particles which could cause HPV disease, could make the persistent long term disease and could finally cause cervical cancer to appear. This FDA born terminology was correct. That any persistent inflammation can produce changes on DNA structure which may turn into cancer has been a knowledge known for hundreds of years. But, the public wishes, the long starving for good news cancer healthcare providers and the public, cut the central part of this paragraph, and used for advertisement only the fist phrase ands the last conclusion – the test that could detect cervical cancer.
This how testing HPV and HPV vaccines became the Holy Grail trophy for most of us.
In order to meet this market requirements, the test providers, BectonDickinson, Quest, Hologic, Qiagen, LabCoprs and the other made another change that indeed discounted the role of the classic Pap test. They proclaim that their tests are better than the Pap test for detecting CIN 2/3 changes. This is not the screening for which the Pap test was developed, this is a diagnosis of an early cervical cancer. It means that the current methods have abandoned the cervical cancer screening principle of the Pap test and compared screening with diagnostic. This is the final strike against the “best cancer screening available.” It went to history.
Suddenly, the Pap test became irrelevant, the screening of cervical cancer eliminated, detection of early signs and the lesion that could develop into cervical cancer were left outside the major examination. The lesions were left to grow and were not eliminated. The environment for punishment was setup.
And the punishment came.
As predicted, the cervical cancer is not giving up so easy. In few years, cervical cancer is coming back and kills women again. Was it necessary to happen? Or, what to do now?
In 2019, CDC projected 13,200 new cases of cervical cancer in US, about4000 more than a couple of years ago. Since the mortality rate is staying the result is in increased number of deaths every year. Is it what we planned and hoped for? No, the reality is just opposite of the hopes.
Based on this information, our conclusion has been that the original Pap test had its problems, but the new technologies introduced as improvements have not done what was expected from them, and the cervical cancer is coming back.
6. Current evidence-based sobering
Now, we have to accept the evidence that the cervical cancer and mortality rate are coming back, due to our inadvertent errors. Cancer is not under our control yet, and it follows it own rules. We have to adapt our strategy, tools and services and to stop its growth, not being exulted by the false hopes. The treatment must follow the natural history, navigate it, but cannot change it without a major, breakthrough, events – which did not happen.
However, the cost of the current Pap test has grown enormously and must be put under control. And kept in range of below $15.00 - $30.00 per test. Our estimate is that this price can be achieved with quantity discount and the new, improved Pap test can be applied globally using the principle, “rich pay for poor.”
7. A light in the tunnel? Maybe!
MarkPap Platform Technology (products and services) in 2019, because available for licensing. It is a new IP comprising of a composite independent utility patent application with 12 revise and derived modules as optional dependent claims indicating to the opportunity to be developed into a set of new individual patents.
To meet the major requirement discussing above the new application shows ability:
EPILOG
The old definition of cancer is still valid: Cancer is an unstoppable growth of abnormal cells, destroying the normal tissue on its path or distribution within the body. This natural history may be affected by many agents, the growth slowed, diverted, affected cell type changed, but the fatal outcome cannot be avoided if the tumor is not entirely eliminated from the body.
This is where we stand now, and what we have to do.
In 1997, immediately after the NIH 1996 Consensus Conference on Cervical cancer hailed the Pap test as “the best anticancer screening test available” but also accusing it of having high false negative readings rate (about 20%,) we asked JNCI “Can Acid Phosphatase Reduce Pap Test False-negative Readings?”[1]
Today, 20 years after this question was posted, and after years of research and experience, our answer is, YES. In our clinical trials, this rate was decreased to 4% or below 5% an acceptable rate of statistical chance of error. {2]
How this is possible? Now, with all medical progress happened in the meantime, the names have changed, the technology has changed, but the real facts have stayed unchallenged.
The fact is that during maturation of cervical epithelium from the basal to superficial squamous layer, the acid phosphatase is changing the isoenzyme structure if the cell is affected by malignancy.
If one can use the principles of PCR and nanomolecular technology applied on live specimen spread on microscopic slide, he would be able to make this affected isoenzyme visible inside a single, morphological identifiable cell, or the tissue sampled while collecting the specimen. This is an Assay for visualization of metabolic changes leading to morphological changes typical for early diagnosis of the epithelial lesion which could lead to the development of cervical cancer. Koilocytosis or HPV disease is the prognostic parameter here.
We applied this Assay in different clinical trials and results were presented on ASCO meetings [2, 3] Later, we have explored how to use this assay in global mass cervical cancer screening and have described the digital image analysis incorporated in an IT networking system connecting scattered POC with expert Medical Centers to receive fast, accurate and low-cost diagnosis and to enable timely intervention – while the patients is on the premises. [4]
This research and experience were published in two editions of our book, What Every Woman Should Know about Cervical Cancer: (Springer 2008 1st Edition, and 2017 2d Edition). [5]
In summary, we have emphasized the role of screening outreach among the women at risk, the opportunity for self-sampling using the new tools, and a new composite biomarker, MEDYKO. This new term represents a combination of letters, ME (metabolic), DY (dysplasia) and KO (koilocytes - HPV disease); altogether, instantly visible under the microscope and amenable for digital imaging and sharing around the world. Details available on www.bioscicon.com [4, 5]
Our new question “Quo Vadis Pap test?” to the interested audience of the Internet readers is whether, using this opportunity, the healthcare providers around the globe could change the strategy for fighting cervical cancer epidemics, and start using the outcome related strategy to Reverse Mortality of the disease in a predicted period of time? This could be the next step in cervical cancer screening.
FIG. 1 MEDYKO a composite biomarker
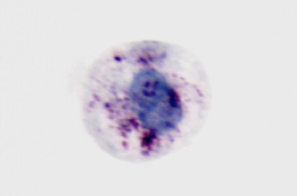
Fig. 1. MarkPap® MEDYKO positive assay. A single cell selected under microscope magnification x40 from the specimen prepared by ThinPrep Processor from the PreservCyt suspension with HPV positive (HC2) solution and with cellular dysplasia combined with koilocytosis. MarkPap Research kit staining. Cervical acid phosphatase isoenzyme is presented by maroon red granules scattered inside the cytoplasm. All three markers indicate LSIL+ diagnosis
List of References:
Cervical cancer is still the leading cause of women's deaths from malignant diseases worldwide. Every year about 600,000 women get cervical cancer and 300,000 die. There are 1.7 billion women at risk in the world, but only 10%, at average, get cervical cancer screening protection.
In developed countries women are much better protected from cervical cancer with regular Pap test screening. For example, 50 million Pap tests are performed each year in the US, a cancer control measure that has dramatically reduced the mortality from this disease. However, there are still 20% women at risk in the US who do not participate. This Global Academy for Women's Health has conducted a survey to assess the reasons why these American women do not get cervical cancer protection although (according to the survey) more than 95% of them are aware of Pap test and have real opportunity to get screening. The most frequent reasons for avoiding the testing were being uncomfortable with the pelvic exam, the cost of the test, and having a stressful time while waiting for the results. However, 95% of women agreed that if some more acceptable variant of Pap test could be introduced, like self-sampling, they would gladly have screening regularly.
The situation is quite different in developing countries. There are still objective barriers for world-wide mass cervical cancer screening, namely cost of the test, lack of infrastructure to perform Pap test the Point-of-Care, no access for testing, fear of testing, lack of knowledge about cervical cancer and the possibility to prevent it (Pap test), uncomfortable pelvic exam and various cultural/religious restrains to visit gynecologist. As a result, in these areas only less than 10% women at risk are protected with some cancer prevention measures. These numbers are changing recently, but most of deaths from cervical cancer are still registered in the low-resource areas of the world. The figure 50% outreach for screening must be achieved in order to have a significant decrease in morbidity and mortality from this preventable disease. Apparently, further improvements of the Pap test are necessary to make it available and affordable for global cervical cancer screening. The Global Academy for Women’s Health hopes to be able to make such a difference.
As a part of its research activity related to the advancement of women’s health, The Academy is closely following and supporting the BioSciCon’s R&D activity on the development of MarkPap® platform technology for cervical cancer control (www.bioscicon.com).
Cervical cancer is still the leading cause of women's deaths from malignant diseases worldwide. Every year about 600,000 women get cervical cancer and 300,000 die. There are 1.7 billion women at risk in the world, but only 10%, at average, get cervical cancer screening protection.
The Global Academy for Women’s Health is particularly interested for those products which can meet the aforementioned unmet needs. These products include (1) MarkPap Test Kit, (2) MarkPap Telecytopathlogy Service, and (3) MarkPap Specimen Self-Collection Kit.
All these tools provide means that, in the near future, a women can take her specimen in the privacy of her home, send it to the local doctor’s office or a small lab where the sample can be processed by a low-trained person who can have instant telecytopathology service for final diagnosis. This is how most of the unmet needs are now much more closer to be met, e.g. cost of the test, lack of infrastructure/professionals at the Pint-of-Care, no access for testing, fear of testing, uncomfortable pelvic exam and various cultural/religious restrains to visit gynecologist. Most important of all the outreach for screening could be markedly increased leading to reduction of morbidity and mortality (11), and the disparity of the healthcare around the world (10). This is the difference that the Global Academy is hoping to make.
Currently, the Global Academy is strongly supporting the development of a local IT Telehealth Center in Rockville, MD, that can provide million diagnostic services per year. The blue print for the Center is ready, space secured and personnel trained. The IT Telehealth Center is expecting State funds in 2012 to finish the final phase of preparation for the start. Actually, the Telehealth Center is expected to be beneficial for the local economy, in-sourcing revenues from abroad, bringing new taxes, building a new infrastructure to stay, retaining jobs and creating new jobs.
In order to succeed, every technology must be accompanied by education. The Global Academy for Women’s Health, Inc. is promoting education of women as the most affordable tool against cervical cancer. The true education is based on facts and the ability to separate reality from wishful thinking. Better education on cervical cancer is a general, still unmet need. Insufficient understanding of this disease may cause unexpected problems - as spreading secondary epidemics. The permanent education is necessary for everybody, even for educators themselves.
Cervical cancer is still the leading cause of women's deaths from malignant diseases worldwide. Every year about 600,000 women get cervical cancer and 300,000 die. There are 1.7 billion women at risk in the world, but only 10%, at average, get cervical cancer screening protection.
In the field of education, the Academy is dedicated to develop high quality professional and public educational programs, organizing and conducting public forums and discussion groups, presenting lectures and workshops, publishing educational and scientific material (brochures and scientific papers), and writing health educational books for general audiences world-wide. Important part is conducting surveys to access public opinion on crucial and controversial issues of women’s health. Surveys could also serve as a tool for validation of the educational program success.
In 2008, Drs. Olivera and Nenad Markovic authored the book "'What every woman should know about cervical cancer", published by an international renown publisher, Springer the book is widely available worldwide as hard cover, paperback, electronic and kindle edition. (Springer.com), or Amazon.com. New, as well as used book can be bought. If you visit those web sites, you will have a chance to read sections of the book directly on the Internet.
This book, providing scientifically proven and medically correct facts should be one of these educational tools that can help women to make their own decision on this and similar dilemmas they may have about cervical cancer prevention, control diagnosis and treatment.. The book summarizes the current views of cervical cancer prevention, control, diagnosis and treatment, presenting them in a language understandable for general public. It is aimed to bring to the readers the rationale for optimism and to provide guidance as how to gain knowledge and skills for critical thinking and making an educated decision when it will be necessary in their lives.
The book is intended to educate the general population of women about true and false information on cervical cancer, HPV infection and vaccines, responsible sexual behavior, other sexually transmitted diseases, cervical cancer detection methods, and the advantages and disadvantages of diagnostic and therapeutic methods as well as holistic medicine available to women worldwide. The purpose of this book is to improve education and reduce risk factors, to improve the awareness of cancer control measures and to improve the outreach of women participating in screening. Its purpose is also to reduce fear of unavoidable death in women who face failure of preventive measures, get cancer and have to fight against the progress of this disease.
However, this book also teaches women about reproductive system and its function, how to increase their level of wellness across all the six dimensions of health, about strategies for stress release applied in health and disease, emphasizing the humane side the fears and despair when women get “bad” results, and where to search for help; precisely, the book is intended to stimulate women to learn more and to actively participate in their treatment--to become advocates of their own health. Authors believe that this book will become a women's companion to turn to when they are motivated to increase their wellness and become healthier, as well as when they are frightened and insecure to learn more and need to diffuse the fear. "Knowledge is power", says one contributor in the "Real People Real Stories" section of this book; she is suggesting that women must learn more to control better their lives. If you wish to read a comprehensive review on this book, please visit Springer’s or Amazon’s web sites, where the book is displayed and offered.
In 2017, the Second, revised, updated and extended edition of the book "What Every Woman Should Know about Cervical Cancer" was published by Springer. The book is available for purchase here.
The Global Academy for Women's Health is planning to open an interactive page on this web site to enable readers of the book to exchange their thoughts, experience and questions. We would appreciate to hear from you whether such a page will be welcome. Please write to us via the e-mail address provided on the contact page.